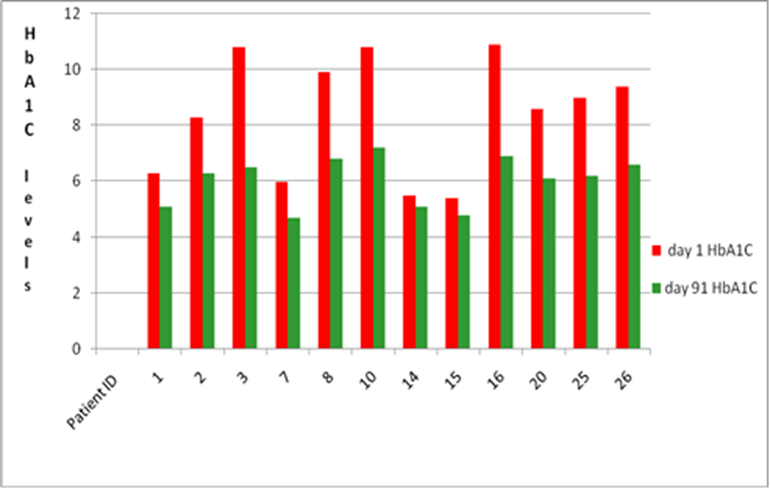
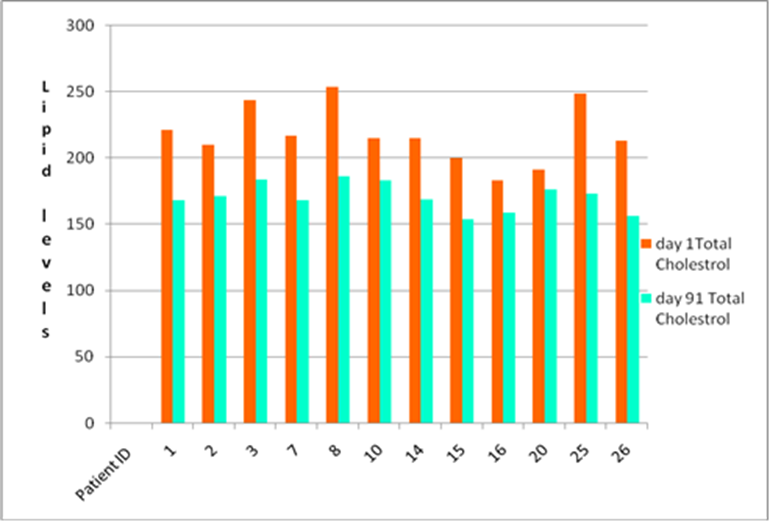
Menon Renewable Products, Inc. has recently concluded a randomized, oral safety and efficacy study of Menon’s latest nutraceutical product in patients with type-II diabetes and hyperlipidemia. Findings indicated that participants who took Menon’s product over 91 days showed improvement to their overall health condition with significant reduction in HbA1C and Total Cholesterol. In contrast, patients in the control group showed increased levels of these parameters by the end of the study.
The study protocol was prepared and approved by an Institutional Review Board and performed by a highly respected clinical trial institute in Chennai, India. A total of 36 adult, human male and female participants were enrolled in the study. The criteria for inclusion included persons aged 18-45 with BMI of 18.5-29.9 kg/m2 and HbA1C levels greater than 8%. Participants were dispensed either Menon’s nutraceutical product or a control product to consume daily with water over a 90 day period. Participants were monitored bi-weekly and blood samples were obtained to measure changes in blood sugar, hemoglobin A1C, triglyceride and cholesterol parameters over the 90 day period.
Results of the study indicated that Menon’s nutraceutical has both anti-diabetic and anti-hyperlipidemic properties. Data demonstrates that the nutraceutical has improved efficacy in the control of elevated blood sugar, HbA1C and cholesterol levels in humans, with an average decrease in A1C of 33.5% over the 90 day period. Total cholesterol levels decreased an average of 21.5%. There were no adverse reactions observed during the trial period.
Menon’s nutraceutical product is scheduled to be released to the market in early 2024 on both Amazon and Walmart marketplaces. To keep informed please follow us on Facebook for all the latest updates: Menon Renewable Products
For more detailed information on this study, please email [email protected]

