L Tran, TC Nhut, KB Alfrey, FT Barrows, D Kuhn and E McLean
Download Article: International Journal of Fisheries and Aqatic Studies
Abstract
Pacific white leg shrimp were stocked (150 PL m-3) into one of four plastic-lined 1000 m3 (0.1 ha) ponds. Ponds were randomly fed one of two diets in duplicate: a commercial shrimp feed (Grobest® Vista Eco), or an open formula, experimental, fishmeal and fish oil-free diet (F3). Shrimp were fed by hand and automatic feeders using commercial feed tables for 56 days. Sub-samples of shrimp (n = 50) were weighed weekly from each pond to evaluate growth and adjust feeding rates. Following harvest, no differences were observed between diets for survival. Combined pond biomass was 2045.0±45.96 kg (S.D.) and 2302.5±28.99 kg for commercial and F3 shrimp respectively. FCR were 1.15±0.00 and 1.03±0.01 (P< 0.05). Individual F3 fed shrimp were significantly (P< 0.05) heavier at trial termination with animals fed the commercial diet weighing 18.03±0.24 g versus 23.25±0.84 g (P< 0.05). Total hemocyte counts were 33.26±0.46 and 33.88±0.72, that for semi-granular cells 20.19±1.65 and 18.72±0.81, granular cells, 15.20±1.39 and 15.56±0.15, hyaline cells, 64.62± 3.01 and 65.72±0.96, and phenoloxidase activity, 56.87±6.12 and 50.29±0.55, for commercial and F3 fed shrimp respectively. A basic economic analysis indicated increased profitability for the F3 fed shrimp (US$7362.43 versus US$10665.62) with a return on investment (ROI) of 64.5% for the commercial feed and 89.8% for the F3 ponds. There were no differences between samples for proximate composition, cooked tail color as assessed by a BASF Salmon Color Fan or texture, as determined by a texture analyzer. Results from this trial unconditionally demonstrate the practicality of cultivating white leg shrimp using marine resource-free-based diets without impacting growth performance or consumer acceptability, while returning increasing ROI.
Introduction
Global food production systems must become more sustainable to ensure the continued supply of high-quality foods and avoid ecosystem destabilization. In this regard a significant challenge for aquaculture will be the reduction or elimination of its addiction to marine resources as feed ingredients. Currently the aquaculture industry uses around 86% of global fishmeal (FM), 73% of fish oil (FO) [1] and most commercially caught krill.[2] However, by 2030 the sector is projected to increase harvest by ~30% above 2018 levels while, over the same timeframe, it has been estimated that FM/O production will only increase by 1 and 7% respectively. [3] Accordingly, if aquaculture is to maintain its current upward production trajectory, it will have to severely reduce or eliminate its reliance on marine resources. [4, 5] For the shrimp feed industry to abandon the habit of using marine resources in prepared diets, alternative feedstuffs must not have any negative consequences to the production performance of the animal. Alternative ingredients must, therefore, be readily available and of invariable quality, known digestibility, satisfying the nutritional needs of the target animal, be competitively priced, and suitable for prevailing processing technologies.[6] Moreover, they must not negatively compromise product quality both from processing and consumer standpoints. The feasibility of meeting the foregoing requirements, while being a big ask, has already been accomplished to a certain extent. Thus, since the beginning of the century there has been a continuous and significant reduction in the amount of FM/O used by shrimp feed manufacturers [1, 5].
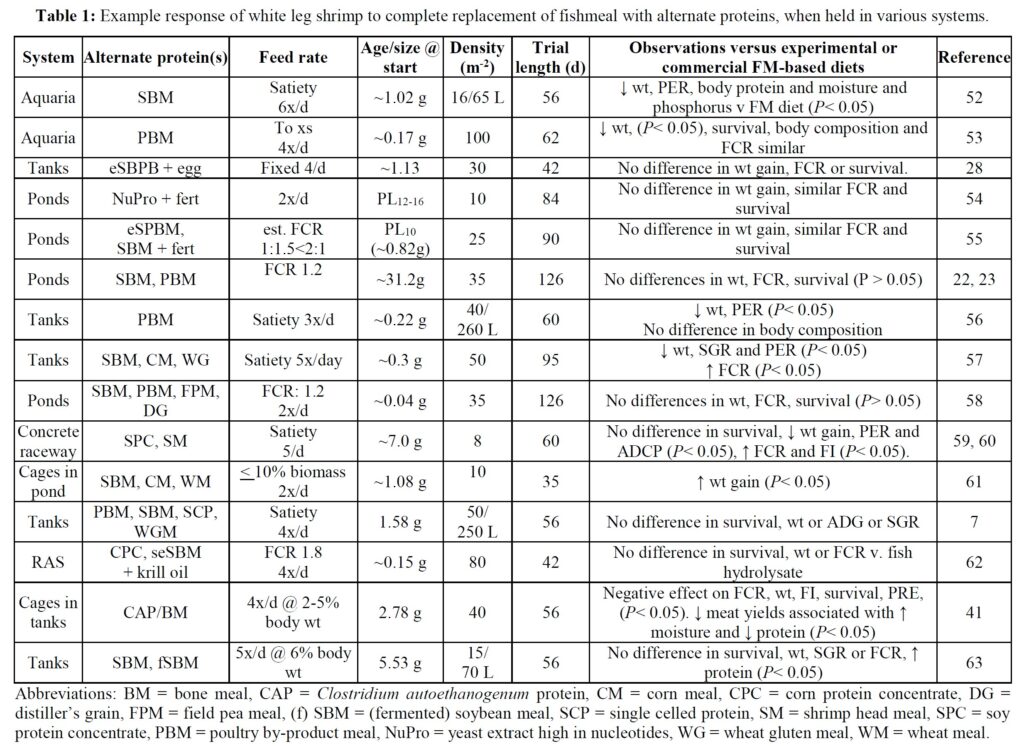
The potential to eliminate marine resources from shrimp feeds is demonstrated by a growing number of laboratory trials that have employed various animal and vegetable protein substitutes for FM (Table 1). In some of these studies the alternative ingredients employed have negatively impacted shrimp growth when compared against FM/O control feeds, especially when a single alternative protein source has been applied. This experience is perhaps not too surprising given that aquafeeds comprise a multitude of ingredients and that a single alternate foodstuff is unlikely to provide the balance of nutrients required by growing animals – i.e., lacks substitutability. [6] In any case, it has been suggested that the scale-up of a single ingredient source, such as poultry by-product, feather and insect meals, while possible in the near-term, is unlikely to satisfy all future demands of the aquafeed sector. [2] When blends of alternate feedstuffs have been used for shrimp diets improved responses have, however, been observed (Table 1). Application of multiple protein and oil sources thus allows the aquafeed manufacturer to replace specific ingredients and avoid supply or demand shocks and accompanying price hikes while minimizing negative nutritional outcomes. [2]
Comparatively few studies have assessed the value of marine resource-free feeds in pond settings (Table 1) and there is a requirement for such practical studies to corroborate laboratory findings. Here, under farm conditions, we compare an open formula FM/O-free feed (F3), validated for its efficiency in the laboratory,[7] against a closed formula, widely used, commercial diet. The objectives of the trial were to assess differences in growth, survival and profitability between feeds, and the impact of diet on body coloration and product quality.
Materials and methods
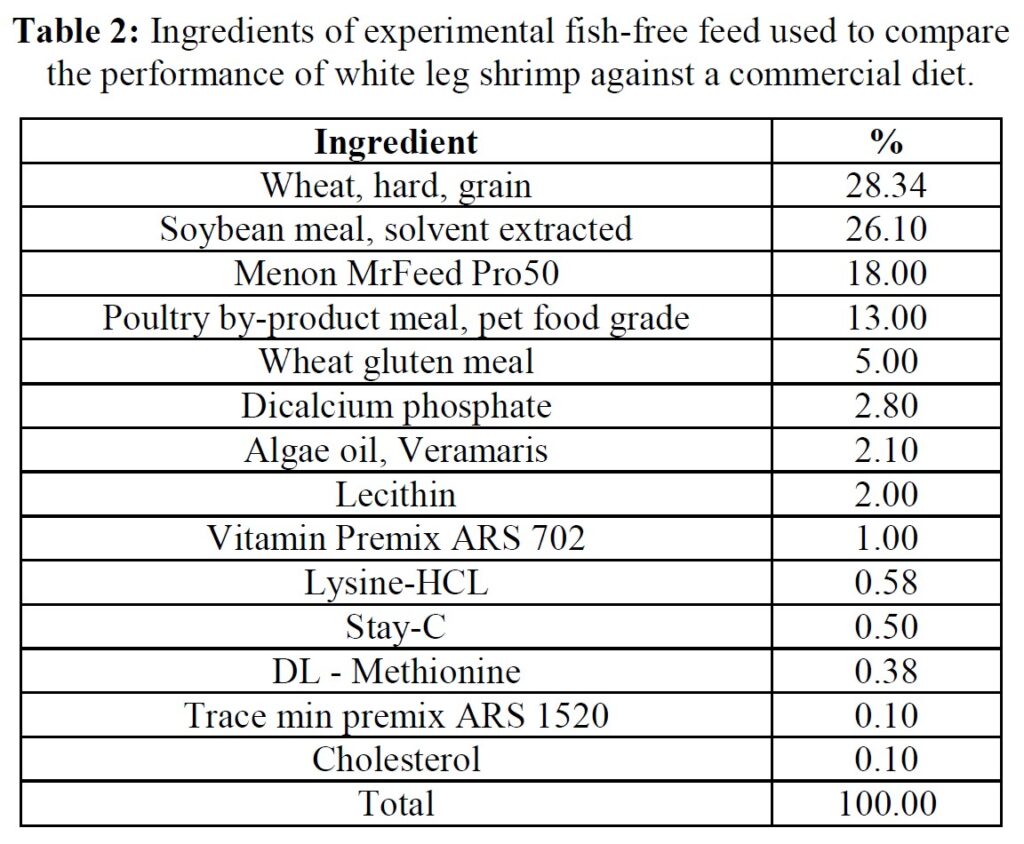
Feeds, animals, and husbandry
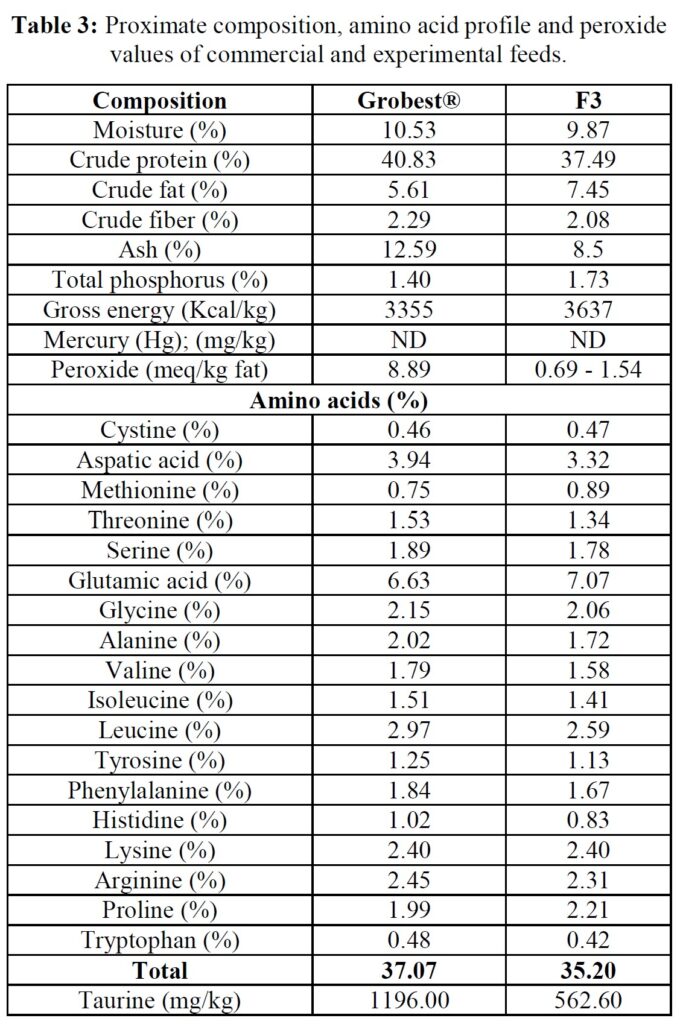
The formula of the open-source experimental diet is presented in Table 2, while proximate and amino acid composition of the commercial (Grobest® Vista Eco) and experimental feeds are presented in Table 3. Shrimp were stocked into four circular plastic-lined ponds (800 m3 capacity) at 150 PL m3 (120,000 pond-1). Ponds were then randomly assigned one of the two diets. Shrimp were fed by hand and automatic feeders using commercial feed tables for 56 days. Sub-samples of shrimp (n = 50) were weighed weekly from each pond to evaluate growth and to adjust feeding rates. Water quality parameters throughout the trial were indistinguishable between ponds and were as follows: temperature: 28.38±0.85 oC; DO2: 6.64±0.12 mg ml-1; pH: 7.83±0.17; salinity: 20.31±2.10 g L-1; alkalinity as CaCO3: 113.50±10.25 mg L-1; TAN 0.85±0.91 mg L-1; nitrite: 15.64±41.07 mg L-1; nitrate: 38.06±48.14 mg L-1. Supplements to each pond included lime: 9.76±5.94 kg; minerals: 3.84±0.86 kg. Water exchange rates were the same across all ponds: 12.43±9.49%. Each pond was aerated using 4 x 7 hp blowers connected to 100 well-distributed Aero-Tubes® and a 3 hp paddlewheel of 9 m length (15 paddles). At trial end shrimp were harvested and a sub-sample of 50 animals taken from each pond for further analyses.
Hemolymph collection and counts
Hemolymph was collected according to the method described by Hernandez-López et al. [8]. The hemolymph was withdrawn from the base of the pleopod of the first abdominal segment near the genital pore. Hemolymph (0.1 mL) was collected into a syringe (26G needle) containing 0.1 mL of precooled (4oC) anticoagulant solution (30 mM trisodium citrate, 340 mM sodium chloride, 115 mM glucose, and 10 mM EDTA at pH 7.55) [9]. The total volume of the hemolymph-anticoagulant (1/1 v/v) mixture was pooled from 10 animals resulting in a 2+ mL volume. Half of this was used for Total Hemocyte Count (THC), and the other for Differential Hemocyte Counts (DHCs). THC employed the method described by Maftuch et al. [10]. While DHC were undertaken according to the method described by Cornick and Stewart [11] as modified by Đặng et al. [12]
Phenoloxidase (PO) activity
Measurement of PO followed the procedure of Hernandez-López et al. [13]. As modified by Yang and Pan [14]. PO activity was assessed following measurement of dopachorme spectrophotometrically using a Thermo Scientific Multiskan Sky High Microplate reader set at 490 nm. The PO reaction was performed on a 96-well plate.
Peroxide value
The peroxide value of the feeds was determined in accordance with the AOAC 965.33/AOCS Cd 8-53 (VF) method. Briefly, samples were dissolved in acetic acid and chloroform solution with the addition of potassium iodide solution in the presence of starch. Iodide was oxidized to iodine by the active oxygen (peroxides) in the product. The liberated iodine was titrated with standardized sodium thiosulfate solution.
Texture and color determinations
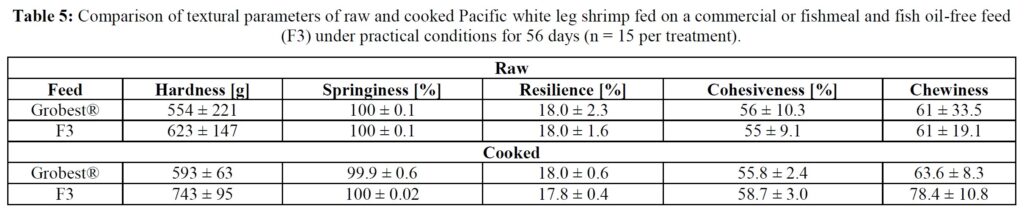
Sample preparation: frozen shrimp were thawed in a refrigerator (< 4 °C) for 24 hours. Shrimp were then deheaded and the tails, with shell on, were boiled in municipal water until the internal temperature reached 63 °C, the temperature recommended by the USFDA to safely consume seafood. Shrimp were immediately strained with water and rinsed for 2 minutes with cold water and subsequently stored in the refrigerator (< 4 °C) for 12 hours. Samples were then brought to room temperature (20 °C) over a two-hour period prior to texture analysis. This ensured that all samples were analyzed at the same temperature. Fifteen cooked shrimp tails from each treatment group were deshelled and analyzed on shrimp tail segment two for texture profile analysis (TPA) using a TA.XT Plus Texture Analyzer outfitted with a 45-degree stainless steel conical TA-15 Probe (Texture Technologies Corp., Hamilton, MA, USA). The TPA method [15] was employed to determine hardness, springiness, resilience, cohesiveness, and chewiness. Color of cooked shrimp was assessed using a BASF Salmon Color Fan.
Statistical analyses
Results were assessed for differences using Student’s t-test. A two-tailed Student’s t-test was used to determine if any differences between treatments were detected for texture. In both cases a P-value of <0.05 was deemed significant.
Results
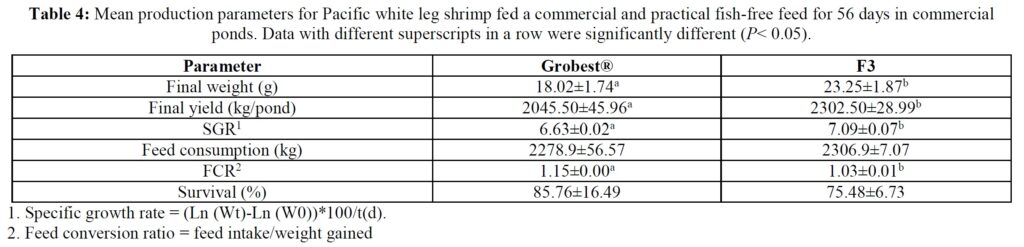
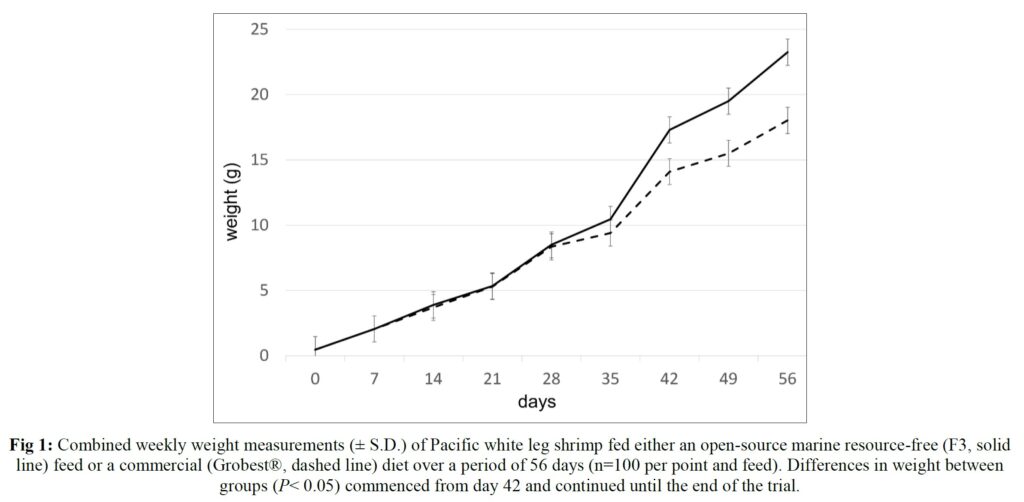
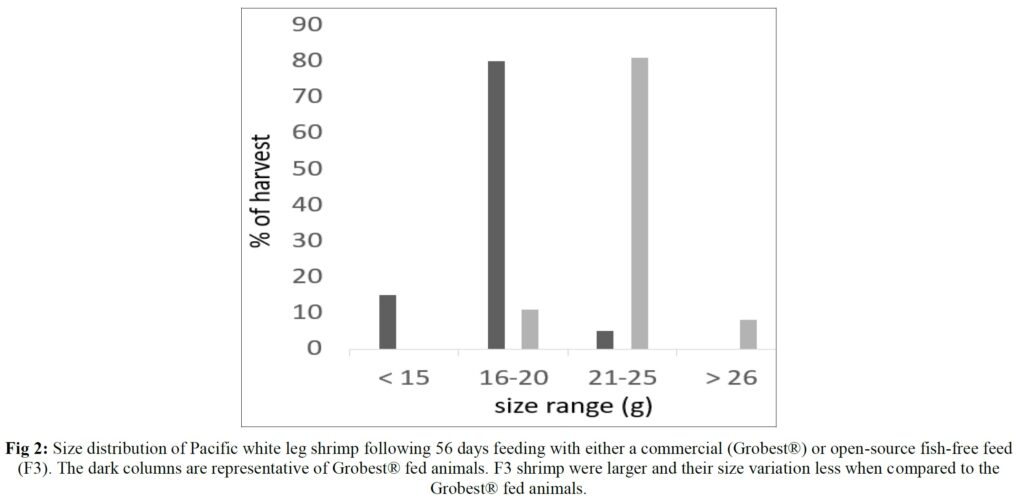
Over the 56-day trial, animals fed the commercial feed returned an average weight of 18.02±1.73 g while shrimp presented with the F3 diet were larger (23.25±1.92 g; P< 0.05; Table 4; Figure 1) and illustrated a tighter size distribution (Figure 2). Specific growth rates were 6.63±0.02 and 7.09±0.07% day-1 (P< 0.05) and final harvest weights 1293.00±318.20 kg and 1502.00±282.84 kg for the commercial and F3 fed ponds respectively (Table 4). Over the study period, there were no differences in feed intake for either diet with 2278.90±56.57 kg of the commercial diet being consumed, while that for the F3 feed was 2306±7.07 kg, yielding feed conversion ratios that differed (P< 0.05) at 1.15±0.00 and 1.03±0.01 respectively (Table 4). At trial
termination animals were inspected for EMS/AHPND, EHP and WSSV (PCR tests) as an integral component of disease monitoring and ponds 2, 3 and 4 were found to be EMS/AHPND positive. However, there were no clinical signs of disease, and no differences were recorded in mean survival rates between treatment groups (Table 4), phenoloxidase activity, THC or percentage hyaline, semi-granular and granular cells.
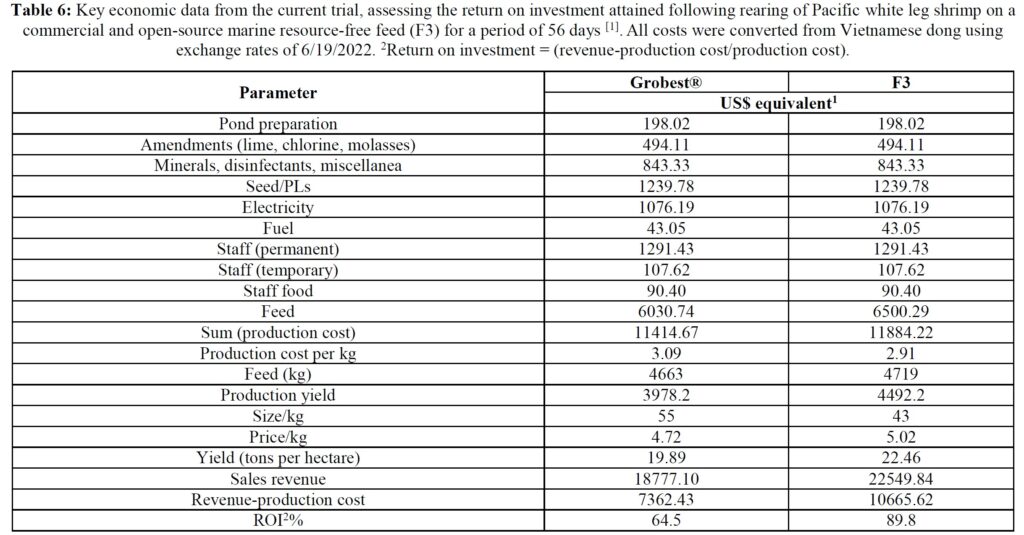
Table 5 summarizes textural analyses of shrimp fed the two different feeds. There were no differences recorded between any of the tested parameters when diets were compared. Table 6 provides an overview of key economic parameters from the current trial, and an assessment of the overall return on investment accrued from each of the two diets investigated. There were no differences in costs associated with the preparation of ponds, their amendments with lime, chlorine, and molasses nor addition of minerals or use of disinfectants throughout the production period. The same was also true for labor costs, power/fuel employed to run pumps, aerators, and for security lighting or other inputs such as post-larvae. Differences were apparent in terms of feed contribution and cost, but this was associated with growth rates and yields returned (Table 6). Greater animal size (Figs. 1 and 2), with concomitant reduction in number of shrimps per kg resulted in the F3 fed animals having a higher value, 6.36% greater than the Grobset® fed stock. Together with the higher production yield, sales revenue was 11.8% higher for the F3 fed shrimp which elicited a more favorable return on investment (Table 6).
Discussion
A plethora of studies have examined the potential for replacing FM/O from white leg shrimp diets. Commonly, single ingredient replacements, for example with a plant protein, have resulted in growth declines but, when blends of alternate proteins have been employed, no, or only limited effects on growth have been recorded, especially when animal proteins are incorporated (Table 1). These, generally laboratory-based trials, have contributed robust evidence to establish there is no need to incorporate marine-derived products in shrimp feeds. However, comparatively few published studies report the performance of shrimp fed marine resource-free diets in commercial-sized ponds. Those that exist are difficult to compare due to variations in animal age/size at trial start, differences in stocking densities employed, variable water quality and temperature regimes, the use of in-pond net cages, differences in feeding schemes and procedures (Table 1), and use of diverse animal stocks, not all of which have been specific pathogen free. Finally, different pond management strategies (e.g., fertilization and inoculation) have been used, which impacts the natural productivity of ponds [16, 17] and thus growth potential of stocked animals.
Irrespective of the differences in trial protocols, production cycle-length pond studies with marine resource-free feeds have generally described equivalent growth to experimental and commercial feeds. For example, Davis et al. [18]. used various diets substituting FM with poultry by-product and soybean meal, pea meal and others, together with Schizochytrium and vegetable oils, and reported weights that were close to those attained by shrimp fed commercial feeds over 62 days at 29 oC. Even with natural pond productivity, however, weight gain observed was less than half that recorded in the present trial. Reid et al. [19] used an organically certifiable yeast-based protein and 1.8 ha ponds inoculated with endemic rotifer and copepods and fertilized with compost. They reported 76-85% survival, and FCRs of ~0.51 over a 12-week period. As observed in the present
trial, shrimp fed the yeast-based diet were larger (19 v. 12 g) and expressed less size variability at harvest than control animals.[20] Nevertheless, growth performance was still poorer than observed in the current study. Other pond trials which have evaluated the impact of eliminating dietary marine resources are slightly flawed because they retain FO, or krill oil, squid meal or fish solubles as feed stimulants [21-26]. Despite these drawbacks, each still provides evidence supportive of eliminating marine resources from shrimp feeds. The present trial, therefore, might be considered preeminent since the feeds employed were truly free of marine-derived ingredients, growth performance attained was superior to that reported in other studies, and survival similar. Growth responses to the marine resource-free diet observed here were similar to others who also used diets in which FM/O were substituted by poultry and soybean meal and Schizochytrium-Mortierella meal [27, 29]. They reported similar growth of shrimp fed experimental and control feeds but used tanks rather than ponds but still attained poorer growth than measured herein, thereby underlining the need to take laboratory studies into the practical environment.
Between-feed differences in growth commenced at week 5 of the trial and to verify whether this resulted due to differences in feed quality, the level of lipid oxidation was examined. FM/O-based feeds can be stored under ambient tropical conditions for up to three months without negative impact on shrimp growth, [30] and since both diets used in the current trial were refrigerated, it would appear unlikely that lipid peroxidation influenced this outcome. Nonetheless, although the F3 diet had a higher lipid content, peroxide value was elevated in the commercial diet, suggesting the onset of oxidation. This may have reflected differences in the lipid type used in each feed. Fish oils, for example, are known to be very sensitive to oxidation due to the presence of high levels of poly-unsaturated fatty acids [31]. In contrast, oils derived from traustochytrids, such as Schizochytrium sp., as used in the F3 feed, are assumed more stable [32]. Feeding shrimp rancid diets can result in increased disease outbreaks and mortality, and reduced growth, poorer feed conversion, and profitability [33]. Reduced growth and inferior feed conversion in shrimp fed the commercial diet used in the present trial might, therefore, have been due to oxidative rancidity influencing palatability and feed conversion. Other studies, however, suggest that the peroxide value of < 9 meq/kg fat recorded for the Grobest® diet was negligible and would be unlikely to impact shrimp performance [34, 35].
Color is an important sensory attribute above that of the purely aesthetic and affects consumer attitudes towards quality and food safety. [36, 37] Indeed, color represents an important facet in setting price, especially when product is sold cooked, fresh, or frozen. [38, 39] Accordingly, given that the food industry is extremely competitive, it is essential that shrimp farmers provide products with minimal color variation. At harvest, shrimp from the present production trial, irrespective of dietary treatment, presented as a typical dark blue/slate, reflecting the color of their rearing environment [40]. In previous studies, replacement of dietary FM has been associated with diminished flesh color following cooking, an occurrence attributed to decreased dietary carotenoid intake. Thus, Yao et al. [41] reported an adverse effect on cooked color when replacing FM with Clostridium autoethanogenum protein. In the present study, however, no differences in color were recorded between the two dietary groups as determined by the salmon color fan. Thus, upon cooking, flesh color transformed into the conventional reddish-orange hue, with no variations in intensity between diets, with samples expressing a dorsal to ventral paling. A null effect on cooked color was also observed when FM was replaced by mealworm Tenebrio molitor. [42] In the current study, the observed lack of effect may have occurred due to additional traces of dietary carotenoids being available from the ponds. As well, the higher dietary lipid levels of the FM/O-free feed might have influenced perceived red coloration [43].
Another important sensory characteristic that influences overall consumer delight, purchasing and repurchasing decisions, is muscle texture. [44] Shrimp are generally described as conveying a tender and delicate texture; a feature that may differ due to diet, rearing environment, and storage. For example, texture measurements increase in western blue shrimp (Litopenaeus stylirostris) as dietary crude protein increases and lipid levels decline [45], observations corroborated by a study in which water boatmen (Trichocorixa sp.) were used as an alternate protein. [46] Storage on ice also changes texture, an effect that may be enhanced due to differences in feed protein quality. [47] Using the same model texture analyzer used here, shrimp have been observed to become dry and rigid following freezing (-18 oC), [48] but these results may reflect incorrect handling since freezing can encourage protein aggregation and dehydration after thawing. Other studies report either no change in texture, or muscle softening after freezing at -20 oC. [49, 50] Textural defects can also occur because of elapsed storage conditions, time between harvest and freezing [51] and method of freezing employed. In the present trial animals were carefully frozen immediately following harvest and it is thus unlikely that samples experienced any serious storage-related degradation.
Conclusion
The present study demonstrates emphatically that Pacific white leg shrimp can be reared on marine resource-free feeds. Moreover, feeds, such as the open-source diet used herein, have no negative effects on shrimp health or quality characteristics. Indeed, superior growth and increased return on investment was realized in this trial, executed under commercial conditions. Further large-scale trials are warranted, with more comprehensive organoleptic assessments, in distinct markets since regional differences in consumer delight for shrimp are evident. Application of our findings, which are supported by a growing body of scientific evidence, provide the shrimp farming sector the means to become environmentally sustainable while nourishing industry growth.
References
- FAO. The state of world fisheries and aquaculture: Towards blue transformation. FAO, Rome, Italy, 2022, 236pp.
- Pelletier N, Klinger DH, Sims NA, Yoshioka J, Kittinger JN. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: joint consideration of potential synergies and trade-offs. Environ. Sci. Technol. 2018;52:5532-5544.
- FAO. The state of world fisheries and aquaculture: Sustainability in action. FAO, Rome, Italy, 2020, 206pp.
- Froehlich HE, Sand Jacobsen N, Essington TE, Clavelle
- T, Halpern BS. Avoiding the ecological limits of forage fish for fed aquaculture. Nat Sustain. 2018;1:298-303.
- Malcorps W, Kok B, van’t Land M, Fritz M, van Doren D, Servin K, et al. The sustainability conundrum of fishmeal substitution by plant ingredients in shrimp feeds. Sustainability. 2019;11:1212.
- Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, et al. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci USA. 2009;106:15103-15110.
- McLean E, Barrows FT, Craig SR, Alfrey K, Tran L. Complete replacement of fishmeal by soybean and poultry meals in Pacific white leg shrimp feeds: Growth and tolerance to EMS/AHPND and WSSV challenge. Aquaculture. 2020;527:735383.
- Hernandez-López J, Gollas-Gollas-Galvan T, Vargas-Albores F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp Biochem Physiol. 1996;113C:61-66.
- Chen YY, Chen JC, Lin YC, Yeh ST, Huang CL. White shrimp Litopenaeus vannamei that have received Gracilaria tenuistipitata extract show early recovery of immune parameters after ammonia stressing. Mar Drugs. 2015;13:3606-3624.
- Maftuch PE, Sudianto A, Rozik M, Nurdiyani R, Sanusi E, Nursyam H, et al. Improvement of innate immune responses and defense activity in tiger shrimp (Penaeus monodon Fab.) by intramuscular administration of the outer membrane protein Vibrio alginolyticus. Springer Plus. 2013;2:1-8.
- Cornick JW, Stewart JE. Lobster (Homarus americanus) hemocytes: Classification, differential counts, and associated agglutinin activity. J Invert Pathol. 1978;31:194-203.
- Đặng Thị Hoàng Oanh, Lê Hữu Thôi và Nguyễn Thanh Phương. Đáp Ứng Miễn Dịch Tự Nhiên Của Tôm Càng Xanh (Macrobrachium Rosenbergii) Cảm Nhiễm Vi-Rút Gây Bệnh Đốm Trắng. Tạp chí Khoa học. 2012;21b:1-9.
- Hernandez-López J, Gollas-Gollas-Galvan T, Vargas-Albores F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp Biochem Physiol. 1996;113C:61-66.
- Yang L, Pan L. Effects of phosphatidyl serine on immune response in the shrimp Litopenaeus vannamei. Cent Eur J Biol. 2013;8:1135-1144.
- Bourne M. Texture Profile Analysis. Food Technology. 1978;32:62-66.
- Moss SM, Divakaran S. Characterization of organic particles associated with rapid growth in juvenile white shrimp, Penaeus vannamei Boone, reared under intensive culture conditions. J Exp Mar Biol Ecol. 1995;187:175-191.
- Moriarty DJW. The role of microorganisms in aquaculture ponds. Aquaculture. 1997;151:333-349.
- Davis DA, Samocha TM, Bullis RA, Patnaik S, Browdy CL, Stokes AD, et al. Practical diets for Litopenaeus vannamei (Boone, 1931): working towards organics and/or all plant production diets. In: Avances en Nutricion Acuıcola VII: Memorias del Septimo Simposium Internacional de Nutricion Acuıcola. 16-19 Noviembre (ed. by L.E. Cruz Suarez, D. RicqueMarie, M.G. Nieto Lopez, D. Villarreal, U. Scholtz & M. Gonzalez), pp. 202-214. Noviembre, Hermosillo, Sonora, Mexico, 2004.
- Reid B, McLean E, Craig SR. Performance
characteristics of shrimp (Litopenaeus vannamei) fed a certified organic feed versus an investigational organic aquafeed. In: Proceedings of the 5th International Conference on Recirculating Aquaculture, July 22-25th, 2004, Roanoke, VA, USA, 2004, 539-542pp. - Craig SR, McLean E. The organic aquaculture movement: a role for NuPro as an alternative protein source. pp. 285-293, In: Nutritional Biotechnology in the Food and Feed Industry: Create, Innovate, Elevate (K. Jacques and P. Lyons, Eds.). Nottingham University Press, 2005.
- Browdy C, Seaborn G, Atwood H, Davis DA, Bullis RA, Samocha TM, Wirth E, et al. Comparison of pond production efficiency, fatty acid profiles, and contaminants in Litopenaeus vannamei fed organic plant-based and fish-meal-based diets. J World Aquacult Soc. 2006;37:437-451.
- Amaya EA, Davis DA, Rouse DB. Replacement of fish meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture. 2007;262:393-401.
- Amaya EA, Davis DA, Rouse DB. Alternative diets for the Pacific white shrimp Litopenaeus vannamei. Aquaculture. 2007;262:419-425.
- Sookying D, Davis DA. Pond production of Pacific white shrimp (Litopenaeus vannamei) fed high levels of soybean meal in various combinations. Aquaculture. 2011;319:141-149.
- García-Ulloa M, Hernandez-Llamas A, de Jesús Armenta-Soto S, Rodríguez-González H. Substituting fishmeal with mixtures of wheat, corn and soya bean meals in diets for the white leg shrimp, Litopenaeus vannamei (Boone): effect on production parameters and preliminary economic assessment. Aquacult Res. 2017;48:4864-4873.
- Ray AJ, Leffler JW, Browdy CL. The effects of a conventional feed versus a fish-free feed and biofloc management on the nutritional and human sensory characteristics of shrimp (Litopenaeus vannamei). Aquacult Int. 2019;27:261-277.
- Patnaik S, Samoocha TM, Davis DA, Bullis RA, Browdy CL. The use of HUFA-rich algal meals in diets for Litopenaeus vannamei. Aquacult Nutr. 2006;12:395-401.
- Samocha TM, Davis DA, Saoud IP, DeBault K. Substitution of fish meal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 2004;231:197-203.
- Samocha TM, Patnaik S, Davis DA, Bullis RA, Browdy CL. Use of commercial fermentation products as a highly unsaturated fatty acid source in practical diets for the Pacific white shrimp Litopenaeus vannamei. Aquacult Res. 2010;41:961-967.
- Laohabanjong R, Tantikitti C, Benjakul S, Supamattaya K, Boonyaratpalin M. Lipid oxidation in fish meal stored under different conditions on growth, feed efficiency and hepatopancreatic cells of black tiger shrimp (Penaeus monodon). Aquaculture. 2009;286:283-289.
- Pike I, Hardy RW. (19 Standards for assessing quality of feed ingredients, pp 473-492, in D’Abramo, L.R., Conklin, D.E., Akiyama, D.M. (Editors), Crustacean nutrition, World Aquaculture Society, Baton Rouge, LA, USA.
- Lv J, Yang X, Ma H, Hu X, Wei Y, Zhou W, et al. The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: Relationship with constituents. Eur J Lipid Sci Technol. 2015;117:1928-1939.
- Bautista MN, Subosa PF. Changes in shrimp feed quality and effects on growth and survival of Penaeus monodon juveniles. Aquaculture. 1997;151:121-129.
- Chen S, Zhuang Z, Yin P, Chen X, Zhang Y, Tian L, et al. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellf Immunol. 2019;88:53-64.
- Yu Y, Liu Y, Yin P, Zhou W, Tian L, Liu Y, et al. Astaxanthin attenuates fish oil-related hepatotoxicity and oxidative insult in juvenile Pacific white Shrimp (Litopenaeus vannamei). Mar Drugs. 2020;18:218.
- Salayo ND, Voon TJP, Selvanathan S. Implicit prices of prawn and shrimp attributes in the Philippine domestic market. Mar Res Econ. 1999;14:65-78.
- Amaya E, Nickell D. Using feed to enhance the color quality of fish and crustaceans, pp. 269-298 in: Davis, D.A. (Editor), and Feeding Practices in Aquaculture. Woodhead Publishing, 2015.
- Wirth FF, Davis KJ. Shrimp purchasing behavior and preferences of seafood dealers. Southern Agricultural Economics Association Annual Meeting, Mobile, Alabama, February. 2002;03;(1-5):15.
- Kritch P, Jintasaporn O, Chumkam S. Effect of background color and nitrite stress on cooked shrimp color of Pacific white shrimp (Litopenaeus vannamei). pp. 10-15, In: Proc 7th Int Conf Food Ag Biotech, July 29-30, Maha Sarakham, Thailand, 2020.
- McLean E. Background color and cultured invertebrates – A review. Aquaculture. 2021;537:736523.
- Yao W, Yang P, Zhang X, Xu X, Zhang C, Li X, et al. Effects of replacing dietary fish meal with Clostridium autoethanogenum protein on growth and flesh quality of Pacific white shrimp (Litopenaeus vannamei). Aquaculture. 2022;549:737770.
- Panini RL, Pinto SS, Nobrega RO, Vieira FN, Francalossi DM, Samuels RI, et al. Effects of dietary replacement of fishmeal by mealworm meal on muscle quality of farmed shrimp Litopenaeus vannamei. Food Res Int. 2017;102:445-450.
- Larsen R, Eilertsen KE, Elvevoll EO. Health benefits of marine foods and ingredients. Biotech Adv. 2011;29:508-518.
- Nunak N, Schleining G. Instrumental textural changes in raw white shrimp during iced storage. J Aq Food Prod Technol. 2011;20:350-360.
- Rivas-Vega ME, Rouzaud-Sandez O, Martinez-Cordova LR, Ezquerrabrauer JM. Effects of feed protein levels on digestive proteolytic activity, texture, and thermal denaturation of muscle protein in reared blue shrimp, J Aquat Food Prod Technol. 2001;10:25-38.
- Martínez-Córdova LR, Campaña-Torres A, Villarreal-Colmenares H, Ezquerra-Brauer JM, Martínez-Porchas M, Cortés-Jacinto E. Evaluation of partial and total replacement of formulated feed by live insects, Trichocorixa sp. (Heteroptera: Corixidae) on the
productive and nutritional response, and postharvest quality of shrimp, Litopenaeus vannamei (Boone 1931). Aquacult Nutr, 2013;19:218-226. - Brauer JME, Leyva JAS, Alvarado LB, Sández OR. Effect of dietary protein on muscle collagen, collagenase and shear force of farmed white shrimp (Litopenaeus vannamei). Eur Food Res Technol. 2003;217:277-280.
- Tsironi T, Dermesonlouoglou E, Giannakourou M, Taoukis P. Shelf life modelling of frozen shrimp at variable temperature conditions. LWT Food Sci Technol. 2009;42:664-671.
- Yamagata M, Low LK. Babana shrimp, Penaeus merguiensis, quality changes during iced and frozen storage. J Food Sci. 1995;60:721-726.
- Balfour S, Badrie N, BadrieIvan N, Chang-Yen I, Chatergoon L. Microbiological, physical and sensory quality of marine shrimp (Peneaus spp.) sold by vendors in Trinidad, West Indies. Int Food Res J. 2014;21:1279-1288.
- Díaz-Tenorio LM, García-Carreño FL, Pacheco-Aguilar R. Comparison of freezing and thawing treatments on muscle properties of white leg shrimp (Litopenaeus vannamei). J Food Biochem. 2007;31:563-576.
- Lim C, Dominy W. Evaluation of soybean meal as a replacement for marine animal protein in diets for shrimp (Penaeus vannamei). Aquaculture. 1990;87:53-63.
- Cheng ZJ, Behnke KC, Dominy WG. Effects of poultry byproduct meal as a substitute for fish meal in diets on growth and body composition of juvenile Pacific white shrimp, Litopenaeus vannamei. J Appl Aquacult. 2002b;12:71-83.
- McLean E, Reid B, Fegan D, Kuhn D, Craig S. Total replacement of fishmeal with an organically certified yeast-based protein in Pacific white shrimp (Litopenaeus vannamei) diets: Laboratory and field trials. Ribarstvo. 2006;64:47-58.
- Browdy C, Seaborn G, Atwood H, Davis DA, Bullis RA, Samocha TM, et al. Comparison of pond production efficiency, fatty acid profiles, and contaminants in Litopenaeus vannamei fed organic plant-based and fish-meal-based diets. J World Aquacult Soc. 2006;37:437-451.
- Chi S, Tan B, Mai K, Zheng S. Growth and feed efficiency of juvenile shrimp Litopenaeus vannamei fed formulated diets containing different levels of poultry by-product meal. J Ocean Univ China. 2009;8:399-408.
- Suárez JA, Gaxiola G, Mendoza R, Cadavid S, Garcia G, Alanis G, Suárez A, Faillace J, Cuzon G. Substitution of fish meal with plant protein sources and energy budget for white shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture. 2009;289:118-123.
- Sookying D, Davis DA. Pond production of Pacific white shrimp (Litopenaeus vannamei) fed high levels of soybean meal in various combinations. Aquaculture. 2011;319:141-149.
- Oujifard A, Seyfabadi J, Kenari AA, Rezaei M. Fish meal replacement with rice protein concentrate in a practical diet for the Pacific white shrimp, Litopenaeus vannamei Boone, 1931. Aquacult Int. 2012b;20:117-129.
- García-Ulloa M, Hernandez-Llamas A, de Jesús Armenta-Soto S, Rodríguez-González H. Substituting fishmeal with mixtures of wheat, corn and soya bean meals in diets for the white leg shrimp, Litopenaeus vannamei (Boone): effect on production parameters and preliminary economic assessment. Aquacult Res. 2017;48:4864-4873.
- Soares R, Peixoto S, Davis RP, Davis DA. Feeding behavior and growth of Litopenaeus vannamei fed soybean-based diets with added feeding effectors. Aquaculture. 2021;536:736487.
- Lin Y.-H, Chen Y.-T. Lactobacillus spp. fermented soybean meal partially substitution to fish meal enhances innate immune responses and nutrient digestibility of white shrimp (Litopenaeus vannamei) fed diet with low fish meal. Aquaculture. 2022;548:737634.
For more information visit www.menon.us.


